Abstract
Background: CAR-T cells have revolutionized the treatment of relapsed B-cell acute lymphoblastic leukemia (ALL), however the field is plagued by 50% relapse, 10-15% non-response, 20% severe toxicity (cytokine release syndrome (CRS) and neurotoxicity) rates. Studies on CAR patient outcomes thus far have largely focused on the contribution of characteristics of the CAR product and post-CAR symptoms 1-8, largely overlooking the potential role of the patient's pre-CAR immunophenotype beyond T-cells in driving therapeutic response and toxicity.
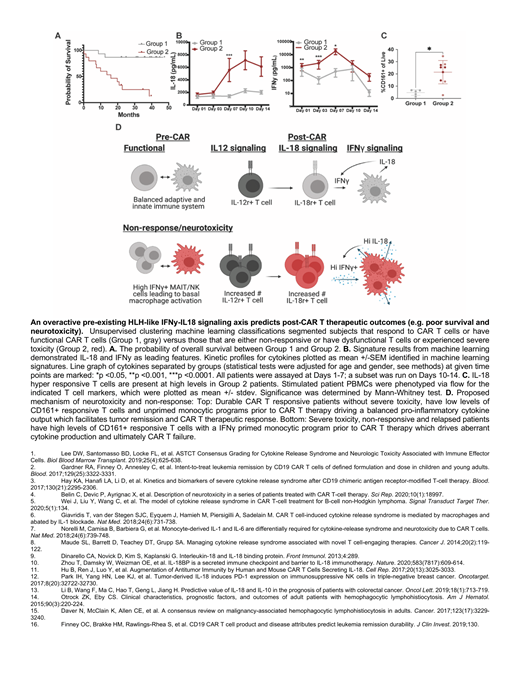
We hypothesized that a patient's inflammatory status prior to immunotherapy could be used clinically to inform interventional and therapeutic mechanistic strategies. Taking inspiration from the clinical presentation overlap between CRS and hemophagocytic lymphohistocytosis (HLH), we hypothesized a common pathophysiology. IL-18 signaling in HLH disorders is well known to play a causative role in anomalous T-cell function 9. Importantly, IL-18 is also linked to tumor regression and progression 10-13. This coupled with the poor overall 5-year survival of secondary HLH patients associated with malignancies 14,15 suggested to us that an overactive IFNγ (T-cell)-IL-18 (myeloid) axis may also drive post-CAR toxicity, as well as overall therapeutic efficacy.
Methods: To test whether patient-specific immunity and an overactive IFNy-IL-18 axis could predict toxicity, response, and survival, we applied a machine learning classifier to cytokine and cell-type profiles of blood samples obtained to prior and at early time points following CD19 CAR T cell infusion of retrospective cohort based on response/non-response, across the continuum of toxicity severity (both CRS and neurotoxicity) and matched availability of samples from 30 of 43 patients on the PLAT phase1 trial (NCT02028455) 2,16.
Results/Discussion: We found evidence for a pre-existing overactive HLH-like axis in some patients; this included both patients with no response to CAR-T therapy as well as patients who experienced high levels of toxicity and diminished long-term survival. Stratification based on this axis was significantly associated with survival rates - Group 2 patients with high levels of IFNy-IL-18 had a median survival of 16.8 months post-CAR, (versus >48 months post-CAR, Group 1). Importantly, we identified an immune sub-type (CD161+IFNy+ T cells which are hyper responsive to IL-18) that is increased in this poor survival Group 2 prior to therapy, which suggests that patients could be effectively stratified prior to CAR infusion.
Given the poor clinical outcome associated with high levels of CD161+IFNy+ T cells, we hypothesized that high levels may be a marker for primed pro-inflammatory myeloid activation, independent of cancer. Thus, we reasoned that donor monocytes from healthy donors with high levels of CD161+IFNy+ T cells could be induced with continued interferon signaling/priming into a Group 2 low survival/neurotoxicity phenotype. Healthy donors with elevated CD161+IFNy+ T cells had increased pro-inflammatory potential including IL-18 signaling in response to IFNy prime. We drove healthy donors to an elevated CD161+ IFNy phenotype utilizing an IL-12 prime, suggesting that patents with high levels of CD161+ cells had a monocytic priming event (e.g., pathogenic exposure, genetic predisposition, vaccination, etc.) prior to therapy that drove the poor clinical outcomes. Based on this data, we propose that a child's cytokine pre-inflammatory status might be independent of cancer and drives immunotherapeutic response and therefore tumor response and toxicity.
Conclusions: In summary, we have identified that an HLH-like signaling axis pre- and early time points post- CAR therapy stratify patient outcomes predicting the onset of poor clinical outcomes prior to their clinical onset such as neurotoxicity, non-response, and relapse. We have also identified biomarker signatures prior and at early time points post-CAR such as cytokines (IFNγ-IL-18 signaling) and immune cell subsets (CD161+ IFNγ+ cells) responsible for the HLH-like signaling axis that could serve as potential future interventional targets. Finally, this study highlights the need to study patient-specific immune characteristics prior to CAR therapy, as these may be predictive of CAR success and failure and may help to identify novel therapeutic approaches and targets improving clinical outcomes.
Burleigh: ShapeTx: Current holder of stock options in a privately-held company. Jensen: BMS: Patents & Royalties; Umoja Biopharma: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bluebird Bio: Research Funding. Masters: IFM therapeutics: Membership on an entity's Board of Directors or advisory committees. Vince: Avammune Therepeutics: Membership on an entity's Board of Directors or advisory committees; Exopharm: Membership on an entity's Board of Directors or advisory committees. Gardner: Novartis: Consultancy; BMS: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal